学術情報:国内の企業・大学の取り組み、背景論文
国内で企業・大学による治験進行中の間葉系幹細胞(mesenchymal stem cell: MSC)等の治療対象
2018.9 NIKKEZI MEDICALより引用
脳梗塞
ヘリオス:他家骨髄由来多能性前駆細胞を点滴静注
ニプロ:自家間葉系幹細胞を培養し点滴静注
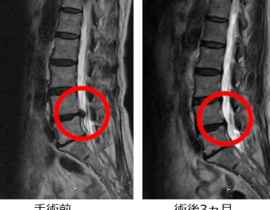
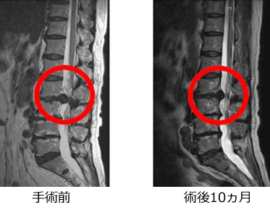
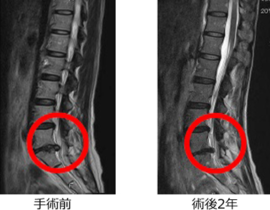
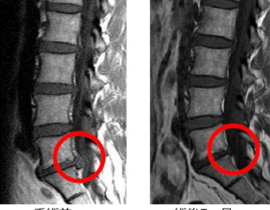
脊髄損傷
ニプロ:自家間葉系幹細胞を培養し点滴静注
2019年2月20日の中央社会保険医療協議会総会で、ニプロの脊髄損傷治療用自己骨髄間葉系幹細胞「ステミラック注」の薬価収載が決定しています。同治療薬は、再生医療等製品版・先駆け審査指定制度の対象製品として初めて、2018年12月に条件・期限付きで承認されたものです。
以下関連情報
https://medical.nikkeibp.co.jp/leaf/mem/pub/report/201902/559944.html
急性心筋梗塞
生命科学インスティチュート:他家Muse細胞を静注
肝硬変
ロート製薬:他家脂肪由来幹細胞を培養し投与
外傷性軟骨及び李男性骨軟骨炎
ツーセル、中外製薬:他家滑膜間葉系幹細胞を培養し移植
半月板損傷
東京医科歯科大学:自家滑膜幹細胞を培養し移植
腹圧性尿失禁
名古屋大学:自家脂肪由来幹細胞を移植
脂肪由来間葉系幹細胞投与の安全性
- ・教科書や学術雑誌上で、間葉系幹細胞の点滴投与・局所投与に関する基礎論文、臨床論文は多数存在し、企業・大学による臨床研究多数進行中です。
- ・当該治療を国内において多くの医療機関が既に提供しており、当該治療に直接起因する重篤な有害事象の報告はありません。
- ・「幹細胞は免疫寛容作用があるので一部では他家移植すら実施されているが特に問題が起こってない点」も考慮すれば、今回の治療は全て自家細胞移植であり、安全性はより大きいと考えられます。
- ・当医療機関で実施するASCの培養法については株式会社バイオ未来工房より技術継承しています。同社は既に前述の国内で間葉系幹細胞を点滴ないしは注射投与による治療を実施している複数の医療機関に同様の細胞培養法の技術継承をし、各医療機関はそれに基づき培養したASCを安全に投与しています。
当クリニックにおいても同様の手法でASC投与を実施するため治療の安全性は担保されます。
背景論文
- Stem Cell Res.2017 8:125 doi: 10.1186/s13287-017-0578-2
Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases.
Lina Zhao, Takerra Johnson, Dong Liu
- Cytotherapy. 2018;20:1143-1154
StemBell therapy stabilizes atherosclerotic plaque after myocardiac infarction.
Linda Woundstra, Jeroen A.M.Belien, Martine C. Morrison, Albert C. Van Rossum, Marco N. Helder, Lynda J.M.Juffermans, Hans W.M. Nissen, Paul A.J.Krijnen
- Stem Cells. 2010;28:1099-1106
A Long-Term Follow-Up Study of Intravenous Autologous Msenchymal Stem Cell
Transplantation in Patients With ischemic Stroke.
Jin Soo Lee, Jt Man Hong, Gyeong Joon Moon, Phil Hyu Lee, Young Hwan Ahn, Oh Young Bang
- Circ Cardiovasc Interv. 2011;4:26-37
Intraarterial Administration of Bone Marrow Mononuclear Cells in Patients With Critical Limb Ischemia: A Randomizes-Start, Placebo-Controlled Pilot Trial(PROVASA).
Dirk H. Walter, Hans Kranskenberg, Jorn O.Balzer, Christooh kalka, Iris Baumgartner, Michael Schluter, Torsten Tonn, Florian Seeger, Stefanie Dimmeler, Edelgard Lindohoff-Last, Andereas M.Zeiher
- Rev Esp Cardiol (Engl Ed). 2015 Jul;68(7):599-611. doi: 10.1016/j.rec.2015.02.025. Epub 2015 May 29.
Adipose-derived Mesenchymal Stem Cells and Their Reparative Potential in Ischemic Heart Disease.
Badimon L, Oñate B, Vilahur G
- Circ J. 2012;76(7):1750-60. Epub 2012 Apr 12.
Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study.
Lee HC1, An SG, Lee HW, Park JS, Cha KS, Hong TJ, Park JH, Lee SY, Kim SP, Kim YD, Chung SW, Bae YC, Shin YB, Kim JI, Jung JS.
- Cell Transplant. 2014;23(4-5):541-7. doi: 10.3727/096368914X678409.
The use of ADSCs as a treatment for chronic stroke.
Chan TM1, Harn HJ, Lin HP, Chiu SC, Lin PC, Wang HI, Ho LI, Chuu CP, Chiou TW, Hsieh AC, Chen YW, Ho WY, Lin SZ.
- Methods in Molecular Biology. 2015;1212:171-181
Potentia Application of Extracellular Vesicles of Human Adipose-Derived Mesenchymal Stem Cells in Alzheimer’s Disease Therapeutics.
Takeshi Katsuda, Katsuyuki Oki, Takahiro Ochiya
- J Pharmacol Sci. 2014;126:293-301
Therapeutic Potential of Human Adipose-Derived Stem Cells in Neurological Disorders.
afety Keun-A Chang, Jun-Ho Lee, Yoo-Hun Suh
- Clin Exp Med. 2016;16:451-461
Human Adipose-Derived Stem Cells Stimulate Neurogeneration.
Ruslan F. Masgutov, Galina A. Masgutova,Margarita N. Zhuravleva, Ilnur I. Salafutodinov, Regina T. Mukhametshina, Yana O. Mukhamedshina, Luchiana M. Lima, Helton J. Reis, Andrey P. Kiyasov, Andras Palotas, Albert A. Rizvanov
- Int J Mol Sci. 2014 Oct 23;15(10):19226-38. doi: 10.3390/ijms151019226.
Stem cell treatment for Alzheimer's disease.
Li M, Guo K, Ikehara S.
- J Pharmacol Sci. 2014;126(4):293-301. doi: 10.1254/jphs.14R10CP. Epub 2014 Nov 18.
Therapeutic potential of human adipose-derived stem cells in neurologicaldisorders.
Chang KA1, Lee JH, Suh YH.
- Pain Physician 2014, 17: E525-E530.
Human umbilical cord mesenchymal stem cell transplantation for the treatment of chronic discogenic low back pain.
Pang X, Yang H, Peng B.
- Transplantation 2011; 92:822-828.
Intervertebral disc repair by autologous mesenchymal bone marrow cells: A pilot study.
Orozco L, Soler R, Morera C, Alberca M, Sánchez A, García-Sancho J.
- Spine (Phila Pa 1976) 2010; 35: E475-E480.
Disc regeneration therapy using marrow mesenchymal cell transplantation: A report of two case studies.
Yoshikawa T, Ueda Y, Miyazaki K, Koizumi M, Takakura Y. - J Pain Res 2014; 7:255-263.
A preliminary report on stem cell therapy for neuropathic pain in humans.
Vickers ER, Karsten E, Flood J, Lilischkis R.
- Pain Med 2015; 16:1475-1481
Pudendal neuralgia: A new option for treatment? Preliminary results on feasibility and efficacy.
Venturi M, Boccasanta P, Lombardi B, Brambilla M, Contessini Avesani E, Vergani C.
- Pain Physician 2008; 11:343-353.
Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells.
Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D.
- Arch Iran Med 2012; 15:422-428.
Intraarticular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis.
Emadedin M, Aghdami N, Taghiyar L, Fazeli R, Moghadasali R, Jahangir S, Farjad R, Baghaban Eslaminejad M.
- Transplantation 2013; 95:1535-1541.
Treatment of knee osteoarthritis with autologous mesenchymal stem cells: A pilot study
Orozco L, Munar A, Soler R, Alberca M, Soler F, Huguet M, Sentís J, Sánchez A, García-Sancho J.
- Transplantation 2014; 97:66-68.
Treatment of knee osteoarthritis with autologous mesenchymal stem cells: Two-year follow-up results.
Orozco L, Munar A, Soler R, Alberca M, Soler F, Huguet M, Sentís J, Sánchez A, García-Sancho J.
- Stem Cells 2014; 32:1254-1266.
Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial.
Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, Ra JC, Oh S, Yoon KS.
- J Bone Joint Surg Am 2014; 96:90-98."
Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: A randomized, double-blind, controlled study
Vangsness CT Jr, Farr J 2nd, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M. - Transplantation 2015; 99:16
Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: A randomized controlled trial.
Vega A, Martín-Ferrero MA, Del Canto F, Alberca M, García V, Munar A, Orozco L, Soler R, Fuertes JJ, Huguet M, Sánchez A, García-Sancho J.
- Case Rep Neurol. 2017 May-Aug;9(2):149-155
Stem cells in the Treatment of Refractory Chronic Migraines.
Alexander Mauskop, Kenneth O.Rothaus
- Stem Cell Research and Therapy 2017;8:262
Safety and tolerability of intradiscal implantation of combined autologous adipose-derived mesenchymal stem cells and hyaluronic acid in patients with chronic discogenic low back pain: 1-year follow up of phase1 study.
Hemant Kumar, Doo-Hoe Ha, Eun-Jong Lee, Jun Hee Park, Jeon Hyun Shim, Tae-Keun Ahn, Kyoung-Tae Kim, Alexnder E. Ropper, Seil Sohn, Chung-Hun Kim, Devang Kashyap Thakor, Soo-Hong Lee, In-Bo Han
- Osteoarthritis and Cartilage. 26(2018):711-29
Osteoarthritis and Stem Cell Therapy in Humans: A Systemic Review.
D.S.Jevotovsky, A.R.Alfonso, T.A. Einhorn, E.S.Chiu
- Circ Res. 2007 May 11;100(9):1249-60.
Adipose-derived stem cells for regenerative medicine.
Gimble JM, Katz AJ, Bunnell BA.
- Circ Res. 2007 May 11;100(9):1249-60.
Adipose-derived stem cells for regenerative medicine.
Gimble JM, Katz AJ, Bunnell BA.
- Cytotherapy. 2015 Sep;17(9):1178-87. doi: 10.1016/j.jcyt.2015.05.007. Epub 2015 Jul 15.
Evaluation ofthe safety and tolerability ofa high-dose intravenous infusion of allogeneic enchymal precursor cells.
McDonald CA, Oehme D, Pham Y, Kelly K, Itescu S, Gibbon A, Jenkin G.